Our Platform
Collaboration. Information. Data exchange.
Accumulus Synergy seeks to accelerate the availability of best-in-class medicines around the world by leveraging breakthrough technology.
Built for the process needs of today and the evolving life sciences–regulatory landscape of the future.
The Accumulus Platform is a first-of-its-kind data and information exchange platform that enhances the current methods of information exchange between life sciences organizations and global health authorities, providing a digital path forward for increased regulatory harmonization. Instead of the traditional dispatch of static information and documents, the platform enables the exchange of information in a secure, cloud-based environment.
Our Platform is a software-as-a-service (SaaS) cloud-based product that centralizes interactions between drug developers and health authorities within a cloud environment and creates an up-to-date, referenceable repository for the exchange of information and data. It also enables real-time exchange of information, including data, as it becomes available throughout the drug development lifecycle. Our Platform is designed to:
- Provide each organization their own space, with content remaining with the originating organization
- Comply with different countries’ security and privacy parameters, ensuring that data is secure and encrypted to the highest degree
- Allow for sharing of select data and information through multi-tenant spaces, with controlled access
- Enable real-time exchange of information including data, as it becomes available throughout the drug development lifecycle

“We can have a faster, more collaborative review process that ideally brings medicines to market faster for patients. It’s always been important; now it’s possible.”
– Hal Stern VP, Head of Technology R&D, at Janssen
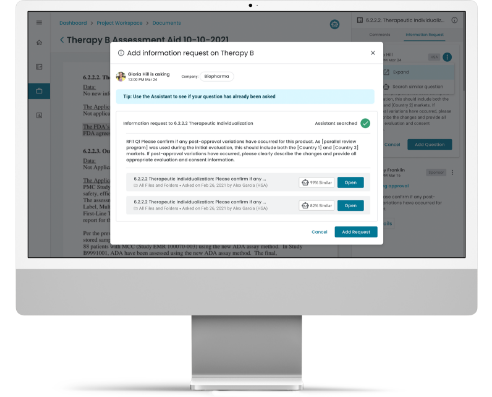
Collaboration Capabilities to Demonstrate Efficiency Improvement.
Our Platform enables enhanced collaboration among regulatory stakeholders in a secure environment to manage data and information within and across organizations.
The Accumulus Platform is also an intuitive communication tool, aimed to centralize and streamline interactions between health authorities and life science organizations.
The Vision for Regulatory Collaboration
- Templated projects
- Collaboration workspaces created per project with non-restrictive combinations of collaborations
- Workflow with dynamic access controls
- Persona-specific real time dashboard
- Calendar-centric project management with milestones and meeting facilitation
- Interactive and dynamic cross health authority collaboration, including health authority invitation processes
- PDF document ingesting/scraping, structured content management support, near real-time collaboration
- Shared information request library powered by natural language processing
Our Approach
Data-Centricity is at the Core of the Accumulus Synergy Platform.
The Accumulus Platform offers innovative technologies that have the capability to enable tremendous progress in the regulatory space.
Our goal is to enhance the current method of information and data exchange by creating a multi-tenant platform that enables efficient insight generation and aids with regulatory convergence. This can facilitate the bilateral exchange between industry and health authorities and foster inter-health authority collaboration, ultimately benefiting patients around the globe. To achieve this, we will focus on delivering a cloud platform that allows for the exchange of structured data in alignment with changing regulatory needs.
The Vision for Data Exchange
The Platform allows users the ability to
- Store validated regulatory data in a common format
- Automatically map terminology across jurisdictions
- Construct templated filings from common data
- Exchange structured filings with global regulators
- Flexibly import/export data through key integrations

We are committed to supporting the development of standards central to solving today’s
interoperability challenges and are a proud member of Health Level Seven International.
“HL7 ” and “Health Level Seven,” are registered trademarks, and the HL7 design is a trademark of Health Level Seven International.
